Oxcarbazepine versus phenytoin monotherapy for epilepsy.
Sarah J Nolan, Martie Muller, Catrin Tudur Smith, Anthony G Marson
Index: Cochrane Database Syst. Rev. 5 , CD003615, (2013)
Full Text: HTML
Abstract
This is an updated version of the original Cochrane review published in The Cochrane Library 2006, Issue 2.Worldwide, phenytoin is a commonly used antiepileptic drug. For the newer drugs such as oxcarbazepine, it is important to know how they compare with standard treatments.To review the best evidence comparing oxcarbazepine and phenytoin when used as monotherapy in participants with partial onset seizures or generalised tonic-clonic seizures with or without other generalised seizure types.We searched the Cochrane Epilepsy Group's Specialised Register (22 January 2013), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2012, Issue 12) and MEDLINE (1946 to 22 January 2013). We handsearched relevant journals and contacted pharmaceutical companies, original trial investigators and experts in the field.Randomised controlled trials in children or adults with partial onset seizures or generalised onset tonic-clonic seizures with a comparison of oxcarbazepine monotherapy with phenytoin monotherapy.This was an individual participant data review. Outcomes were time to (a) treatment withdrawal (b) 12-month remission (c) six-month remission and (d) first seizure post randomisation. We used Cox proportional hazards models to obtain study-specific estimates of hazard ratios (HR) with 95% confidence intervals (CI) with the generic inverse variance method used to obtain the overall pooled HR and 95% CI.Individual participant data were available for 480 out of 517 participants (93%) from three included trials. For remission outcomes, a HR > 1 indicates an advantage to phenytoin and for first seizure and withdrawal outcomes a HR > 1 indicates an advantage to oxcarbazepine.The main overall results (pooled HR, 95% CI) were: (i) time to withdrawal of allocated treatment 1.65 (1.08 to 2.52), (ii) time to 12-month remission 0.92 (0.68 to 1.24), (iii) time to six-month remission 0.90 (0.70 to 1.15), (iv) time to first seizure 1.07 (0.83 to 1.39). Results indicate a statistically significant advantage for oxcarbazepine over phenytoin for time to treatment withdrawal, but insufficient evidence to suggest a difference between the drugs for other outcomes. By epilepsy type, there is no significant advantage for either drug for generalised epilepsy, however there is a significant advantage for partial epilepsy with oxcarbazepine for time to treatment withdrawal (HR 1.95; 95% CI 1.15 to 3.33).For participants with partial onset seizures oxcarbazepine is significantly less likely to be withdrawn, but current data do not allow a statement as to whether oxcarbazepine is equivalent, superior or inferior to phenytoin in terms of seizure control. However, the design of the studies may have biased seizure outcomes and misclassification of epilepsy type may have biased withdrawal rates.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
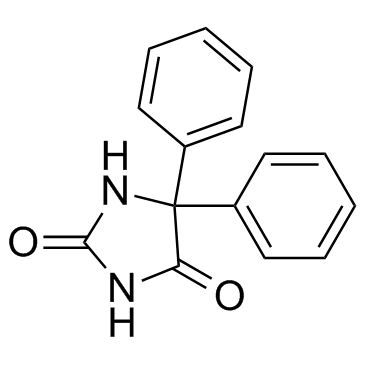 |
phenytoin
CAS:57-41-0 |
C15H12N2O2 | |
 |
Oxcarbazepine
CAS:28721-07-5 |
C15H12N2O2 |
|
The effect of chronic phenytoin administration on single pro...
2015-01-01 [Psychopharmacol. Ser. 232(1) , 47-56, (2015)] |
|
Evaluation of thein vitro/in vivodrug interaction potential ...
2014-06-01 [Food Chem. Toxicol. 68 , 117-27, (2014)] |
|
Metabolic drug-drug interaction potential of macrolactin A a...
2014-09-01 [Antimicrob. Agents Chemother. 58(9) , 5036-46, (2014)] |
|
Role of hepatic blood flow and metabolism in the pharmacokin...
2014-12-01 [Xenobiotica 44(12) , 1108-16, (2014)] |
|
Preparation and evaluation of high dispersion stable nanocry...
2014-11-01 [J. Pharm. Sci. 103(11) , 3772-81, (2014)] |