| Structure | Name/CAS No. | Articles |
|---|---|---|
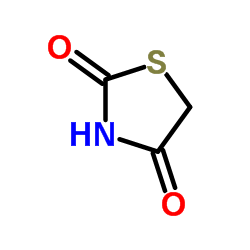 |
2,4-Thiazolidinedione
CAS:2295-31-0 |
|
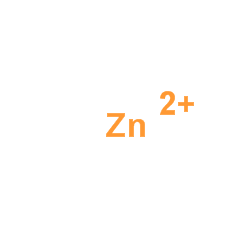 |
ALPHA-MANNOSIDASE
CAS:9025-42-7 |
|
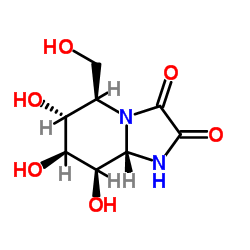 |
Kifunensine
CAS:109944-15-2 |
|
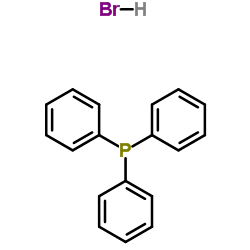 |
Triphenylphosphine hydrobromide (1:1)
CAS:6399-81-1 |
|
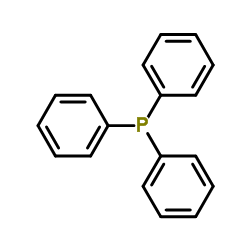 |
Triphenylphosphine
CAS:603-35-0 |