| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(S)-(-)-Limonene
CAS:5989-54-8 |
|
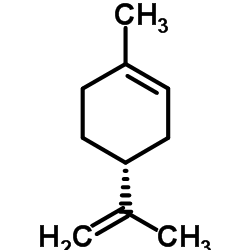 |
(+)-Limonene
CAS:5989-27-5 |
|
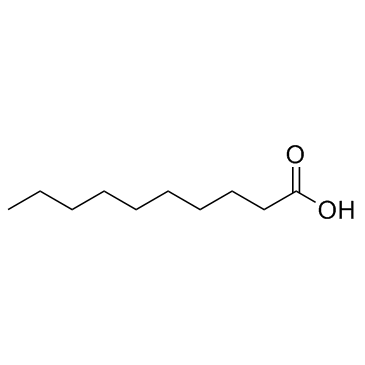 |
Decanoic acid
CAS:334-48-5 |
|
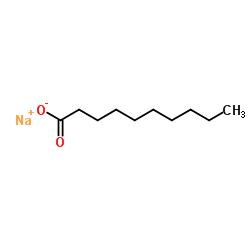 |
Sodium decanoate
CAS:1002-62-6 |
|
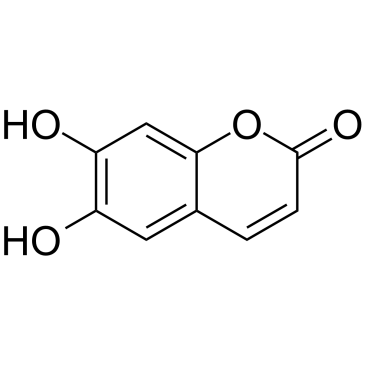 |
6,7-Dihydroxycoumarin
CAS:305-01-1 |
|
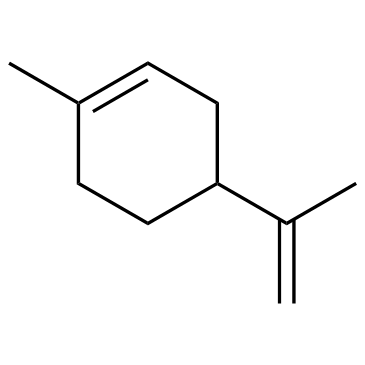 |
limonene
CAS:138-86-3 |