| Structure | Name/CAS No. | Articles |
|---|---|---|
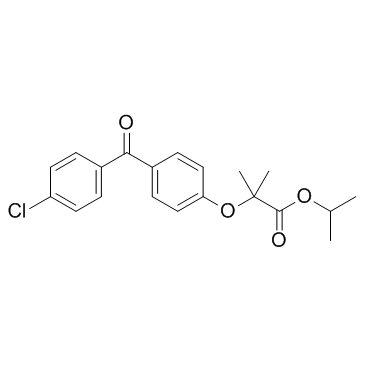 |
Fenofibrate
CAS:49562-28-9 |
|
 |
Lysozyme
CAS:12650-88-3 |
|
 |
Lysozyme
CAS:9001-63-2 |
|
 |
Lysozyme hydrochloride
CAS:9066-59-5 |
|
 |
Human milk lysozyme
CAS:12671-19-1 |
|
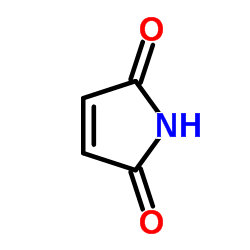 |
Maleimide
CAS:541-59-3 |