| Structure | Name/CAS No. | Articles |
|---|---|---|
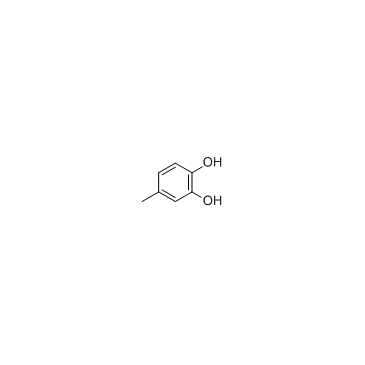 |
4-methylcatechol
CAS:452-86-8 |
|
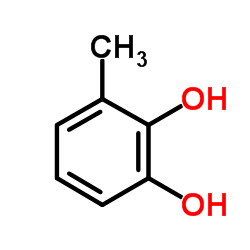 |
3-Methylcatechol
CAS:488-17-5 |
|
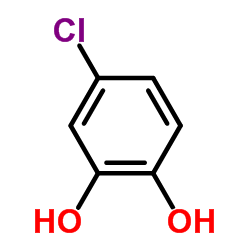 |
4-Chlorocatechol
CAS:2138-22-9 |