| Structure | Name/CAS No. | Articles |
|---|---|---|
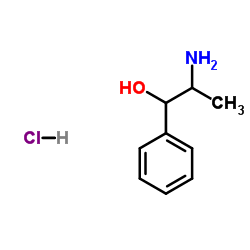 |
DL-Norephedrine hydrochloride
CAS:154-41-6 |
|
 |
(-)-norephedrine
CAS:492-41-1 |
|
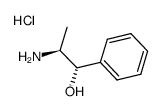 |
(1S,2S)-2-amino-1-phenylpropan-1-ol,hydrochloride
CAS:2153-98-2 |
|
 |
Cathine
CAS:492-39-7 |
|
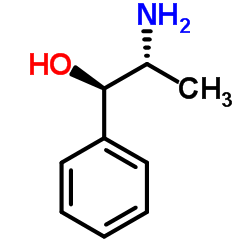 |
L-(−)-Norpseudoephedrine
CAS:37577-07-4 |
|
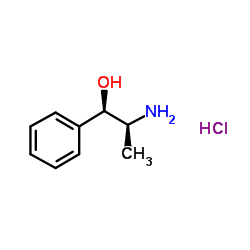 |
(1S,2R)-(+)-Norephedrine
CAS:37577-28-9 |
|
 |
(+/-)-n-ethylamphetamine
CAS:457-87-4 |
|
 |
pseudoephedrine
CAS:90-82-4 |