| Structure | Name/CAS No. | Articles |
|---|---|---|
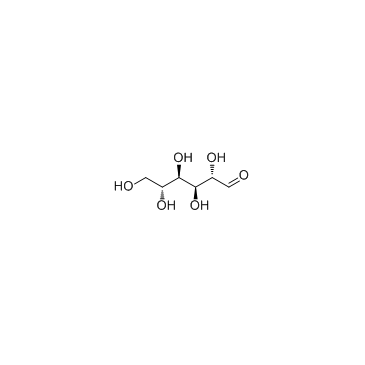 |
D-Mannose
CAS:3458-28-4 |
|
 |
6-Aminohexyl–Agarose
CAS:58856-73-8 |