| Structure | Name/CAS No. | Articles |
|---|---|---|
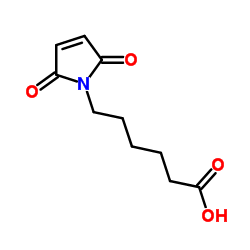 |
6-Maleimidocapronic acid
CAS:55750-53-3 |
|
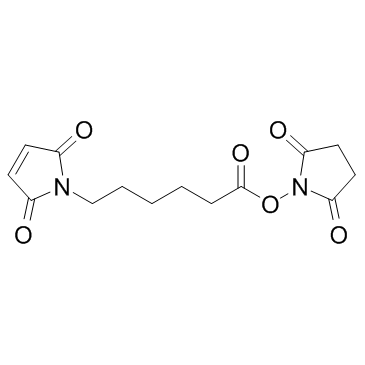 |
N-Succinimidyl 6-maleimidohexanoate
CAS:55750-63-5 |