| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Pentobarbital
CAS:76-74-4 |
|
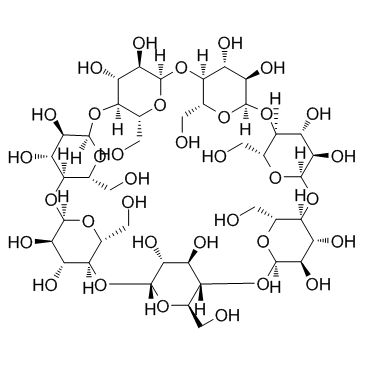 |
β-cyclodextrin
CAS:7585-39-9 |
|
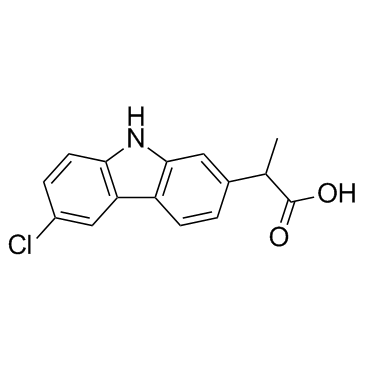 |
Carprofen
CAS:53716-49-7 |
|
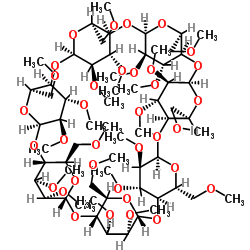 |
Heptakis(2,3,6-tri-O-methyl)-b-cyclodextrin
CAS:55216-11-0 |