Ab initio molecular electrostatic potentials of perillartine analogues: implications for sweet-taste receptor recognition.
T J Venanzi, C A Venanzi
Index: J. Med. Chem. 31(10) , 1879-85, (1988)
Full Text: HTML
Abstract
A model for the recognition of the perillartine analogues has been determined from a consideration of the molecular electrostatic potentials calculated at the ab initio 3-21G level for a select set of biologically active analogues. The model stresses the importance of two regions of negative electrostatic potential. One region, near the oxime moiety, does not vary in shape or value with substitution in the hydrocarbon domain. A second region in the hydrocarbon domain varies in depth, extension, orientation, and shape, depending on the nature of the substituent. The depth, relative position, and orientation of this latter region in the most potent systems (the 1,4-cyclohexadiene analogue and its p-methyl derivative) serve as the basis for the optimum recognition pattern of these analogues. The rank order of taste potencies is in general agreement with predictions based on this model. In addition, some conclusions are drawn concerning the receptor-analogue interaction as well as the electrostatic features of the receptor.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
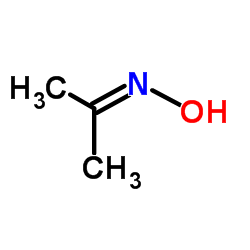 |
Propan-2-one oxime
CAS:127-06-0 |
C3H7NO |
|
Slow oxidation of acetoxime and methylethyl ketoxime to the ...
1999-02-01 [Toxicol. Sci. 47(2) , 144-50, (1999)] |
|
Synthesis and anticancer evaluation of benzyloxyurea derivat...
2014-01-01 [Chem. Pharm. Bull. 62(9) , 898-905, (2014)] |
|
Reversion of structure-activity relationships of antitumor p...
2007-01-01 [Mol. Pharmacol. 71(1) , 357-65, (2007)] |
|
Amination of tyrosine in liver cytosol protein of male F344 ...
1997-12-01 [Chem. Res. Toxicol. 10(12) , 1420-6, (1997)] |
|
Sex and organ differences in oxidative DNA and RNA damage du...
1990-09-01 [Carcinogenesis 11(9) , 1659-62, (1990)] |