| Structure | Name/CAS No. | Articles |
|---|---|---|
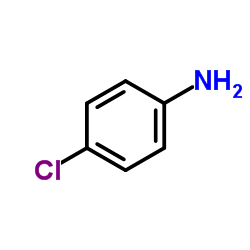 |
4-Chloroaniline
CAS:106-47-8 |
|
 |
4-AMINOPYRIDINE
CAS:504-24-5 |
|
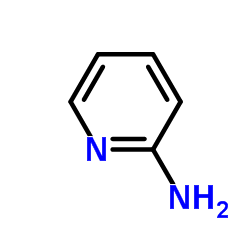 |
2-Aminopyridine
CAS:504-29-0 |
|
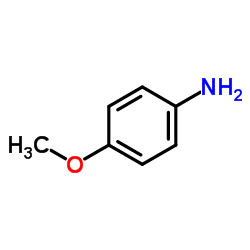 |
p-Anisidine
CAS:104-94-9 |
|
 |
Aniline
CAS:62-53-3 |