| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
0MPTP hydrochloride
CAS:23007-85-4 |
|
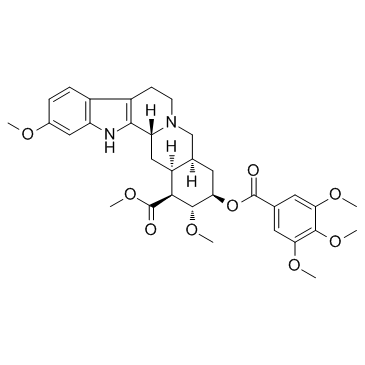 |
Reserpine
CAS:50-55-5 |
|
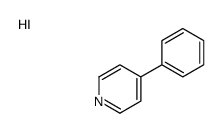 |
MPP+ iodide
CAS:36913-39-0 |