| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Formate dehydrogenase
CAS:9028-85-7 |
|
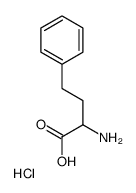 |
l-homophenylalanine hydrochloride salt
CAS:21176-60-3 |