| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甲酸酯脱氢酶
CAS:9028-85-7 |
|
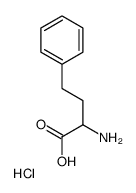 |
L-苯基丁氨酸 盐酸盐
CAS:21176-60-3 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
甲酸酯脱氢酶
CAS:9028-85-7 |
|
 |
L-苯基丁氨酸 盐酸盐
CAS:21176-60-3 |