Organic Letters
2009-03-05
Synthesis of gamma-amino esters via Mn-mediated radical addition to chiral gamma-hydrazonoesters.
Gregory K Friestad, Koushik Banerjee
Index: Org. Lett. 11(5) , 1095-8, (2009)
Full Text: HTML
Abstract
Highly stereoselective Mn-mediated couplings of alkyl iodides with chiral N-acylhydrazones bearing ester functionality afford a series of gamma-hydrazino esters, including gamma-substituted, alpha,gamma-disubstituted, and alpha,alpha,gamma-trisubstituted examples. In contrast to prior work with chiral N-acylhydrazones, high stereoselectivity was observed even in the absence of Lewis acid. Microwave-assisted acylation with trifluoroacetic anhydride and reductive N-N bond cleavage provided the gamma-amino ester functionality in a synthetically useful N-TFA-protected form.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
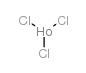 |
Indium chloride
CAS:10025-82-8 |
Cl3Ho |
Related Articles:
More...
|
Synthesis of CuInSe(2) nanocrystals with trigonal pyramidal ...
2009-03-11 [J. Am. Chem. Soc. 131(9) , 3134-5, (2009)] |
|
Pleural effects of indium phosphide in B6C3F1 mice: nonfibro...
2009-12-01 [Exp. Lung Res. 35(10) , 858-82, (2009)] |
|
InCl3 mediated one-pot multicomponent synthesis, anti-microb...
2010-09-01 [Bioorg. Med. Chem. Lett. 20 , 5054-61, (2010)] |
|
InCl(3)-catalyzed asymmetric aza-Friedel-Crafts reaction of ...
2010-08-16 [Carbohydr. Res. 345 , 1708-1712, (2010)] |
|
InCl3-catalyzed allylic Friedel-Crafts reactions toward the ...
2010-11-05 [Org. Lett. 12(21) , 4884-7, (2010)] |