| Structure | Name/CAS No. | Articles |
|---|---|---|
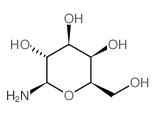 |
1-amino-1-deoxy-beta-d-galactose
CAS:74867-91-7 |
|
 |
Indium chloride
CAS:10025-82-8 |