| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
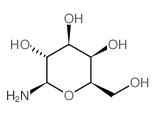 |
1-氨基-1-脱氧-β-D-半乳糖
CAS:74867-91-7 |
|
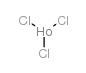 |
氯化铟
CAS:10025-82-8 |