| Structure | Name/CAS No. | Articles |
|---|---|---|
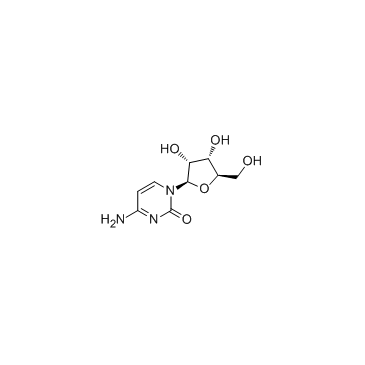 |
Cytidine
CAS:65-46-3 |
|
 |
2'-Deoxycytidine-5'-monophosphoric acid
CAS:1032-65-1 |
|
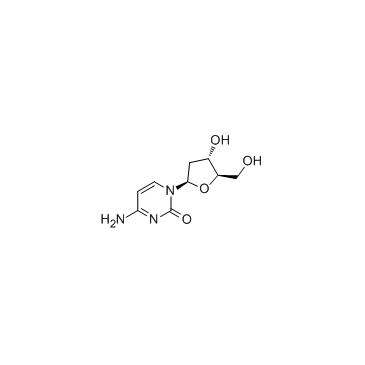 |
2'-Deoxycytidine monohydrate
CAS:951-77-9 |