| Structure | Name/CAS No. | Articles |
|---|---|---|
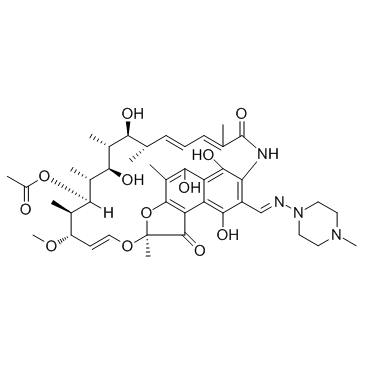 |
Rifampicin
CAS:13292-46-1 |
|
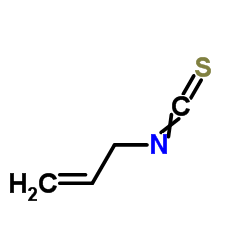 |
Allyl isothiocyanate
CAS:57-06-7 |
|
 |
Isoniazid
CAS:54-85-3 |
|
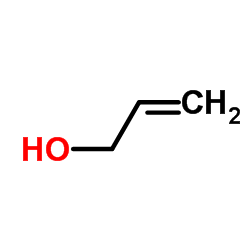 |
Allyl alcohol
CAS:107-18-6 |