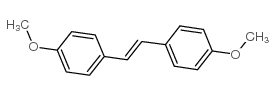
|
~% |
|
~% |
|
~% |
|
~% |
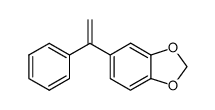
![5-[(Z)-2-phenylethenyl]benzo[1,3]dioxole Structure](https://image.chemsrc.com/caspic/494/51003-16-8.png)
![1ξ-ethoxy-1ξ-benzo[1,3]dioxol-5-yl-propene Structure](https://image.chemsrc.com/caspic/277/108757-52-4.png)

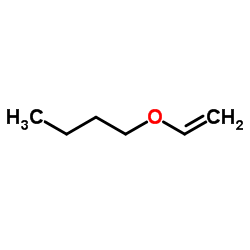
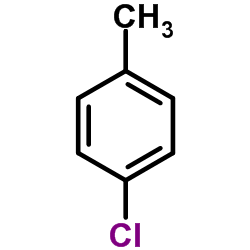

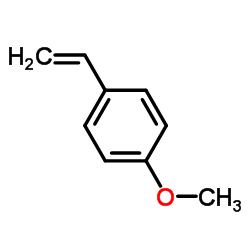
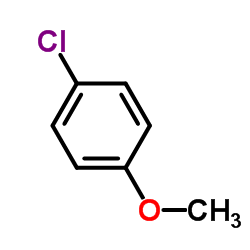
![1-methoxy-4-[1-(4-methoxyphenyl)ethenyl]benzene Structure](https://image.chemsrc.com/caspic/183/4356-69-8.png)