| Structure | Name/CAS No. | Articles |
|---|---|---|
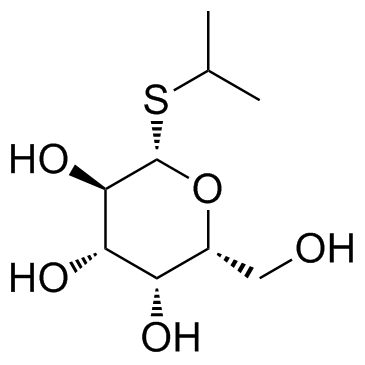 |
Isopropyl-beta-D-thiogalactopyranoside
CAS:367-93-1 |
|
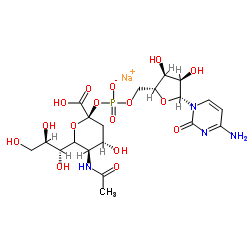 |
Cytidine 5'-monophosphate-N-acetylneuraminic acid
CAS:3063-71-6 |