| Structure | Name/CAS No. | Articles |
|---|---|---|
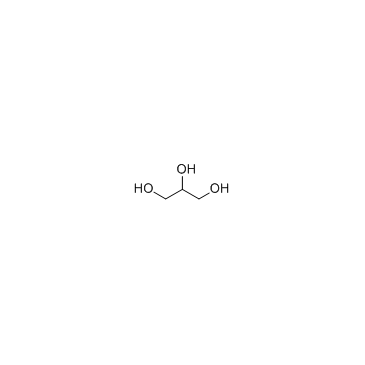 |
Glycerol
CAS:56-81-5 |
|
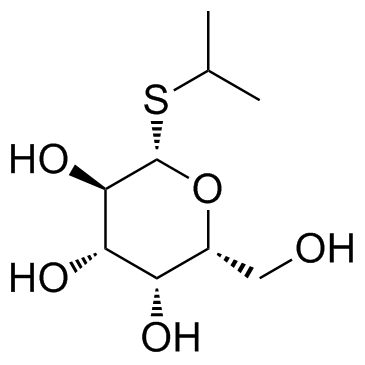 |
Isopropyl-beta-D-thiogalactopyranoside
CAS:367-93-1 |