Effect of fluorine position on the coordinating ability of fluorosalicylic acids--an experimental study complemented with computations.
Terézia Szabó-Plánka, Béla Gyurcsik, István Pálinkó, Nóra Veronika Nagy, Antal Rockenbauer, Rastislav Sípoš, Jozef Sima, Milan Melník
Index: J. Inorg. Biochem. 105(1) , 75-83, (2011)
Full Text: HTML
Abstract
The complexation of 3-, 4-, and 6-fluorosalicylic acids (HL) with copper(II) was investigated in aqueous solution by pH-potentiometry combined with UV-visible spectrophotometry, and in 50 v/v % water-methanol mixture by the two-dimensional ESR simulation method. Both methods showed the formation of [CuLH(-1)] and [CuL(2)H(-2)](2-) of high stabilities, and, at low excess of ligand, the ESR-silent mixed hydroxido complex [Cu(2)L(2)H(-3)](-). Further species were also identified by the two-dimensional ESR simulation method: [CuL](+) in the acidic region, the minor dimer [Cu(2)L(2)H(-2)], and the cis and the trans isomers for [CuL(2)H(-2)](2-). The position of the fluorine atom in the aromatic ring had significant effect on the coordination abilities of the ligands, in good correlation with their reported biological activities. It was 3-fluorosalicylic acid, which formed the most stable complexes [CuLH(-1)] and [CuL(2)H(-2)](2-), while the mononuclear complexes with 6-fluorosalicylic acid were found to be the least stable. For the other ligands (including 5-fluorosalicylic acid studied recently), complexes of medium stabilities were formed. For the interpretation of these findings, ab initio and semi-empirical quantum chemical calculations were carried out for the ligand molecules, isolated and surrounded by water molecules, respectively.Copyright © 2010 Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
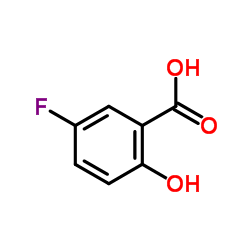 |
5-Fluoro-2-hydroxybenzoic acid
CAS:345-16-4 |
C7H5FO3 |
|
Identifying chelators for metalloprotein inhibitors using a ...
2011-01-27 [J. Med. Chem. 54 , 591-602, (2011)] |
|
Quantification of free and total salicylic acid in plants by...
2005-08-01 [Anal. Bioanal. Chem 382(7) , 1620-7, (2005)] |
|
Enzymatically amplified time-resolved fluorescence immunoass...
1992-02-15 [Anal. Chem. 64(4) , 342-6, (1992)] |
|
Cα-C bond cleavage of the peptide backbone in MALDI in-sourc...
2011-07-01 [J. Am. Soc. Mass Spectrom. 22(7) , 1224-33, (2011)] |
|
Mono- and disalicylic acid derivatives: PTP1B inhibitors as ...
2007-10-15 [Bioorg. Med. Chem. 15 , 6535-48, (2007)] |