Cα-C bond cleavage of the peptide backbone in MALDI in-source decay using salicylic acid derivative matrices.
Daiki Asakawa, Mitsuo Takayama
Index: J. Am. Soc. Mass Spectrom. 22(7) , 1224-33, (2011)
Full Text: HTML
Abstract
The use of 5-formylsalicylic acid (5-FSA) and 5-nitrosalicylic acid (5-NSA) as novel matrices for in-source decay (ISD) of peptides in matrix-assisted laser desorption/ionization (MALDI) is described. The use of 5-FSA and 5-NSA generated a- and x-series ions accompanied by oxidized peptides [M - 2 H + H](+). The preferential formation of a- and x-series ions was found to be dependent on the hydrogen-accepting ability of matrix. The hydrogen-accepting ability estimated from the ratio of signal intensity of oxidized product [M - 2 H + H](+) to that of non-oxidized protonated molecule [M + H](+) of peptide was of the order 5-NSA > 5-FSA > 5-aminosalicylic acid (5-ASA) ≒ 2,5-dihydroxyl benzoic acid (2,5-DHB) ≒ 0. The results suggest that the hydrogen transfer reaction from peptide to 5-FSA and 5-NSA occurs during the MALDI-ISD processes. The hydrogen abstraction from peptides results in the formation of oxidized peptides containing a radical site on the amide nitrogen with subsequent radical-induced cleavage at the Cα-C bond, leading to the formation of a- and x-series ions. The most significant feature of MALDI-ISD with 5-FSA and 5-NSA is the specific cleavage of the Cα-C bond of the peptide backbone without degradation of side-chain and post-translational modifications (PTM). The matrix provides a useful complementary method to conventional MALDI-ISD for amino acid sequencing and site localization of PTMs in peptides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
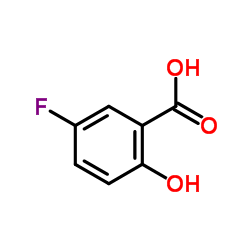 |
5-Fluoro-2-hydroxybenzoic acid
CAS:345-16-4 |
C7H5FO3 |
|
Identifying chelators for metalloprotein inhibitors using a ...
2011-01-27 [J. Med. Chem. 54 , 591-602, (2011)] |
|
Quantification of free and total salicylic acid in plants by...
2005-08-01 [Anal. Bioanal. Chem 382(7) , 1620-7, (2005)] |
|
Enzymatically amplified time-resolved fluorescence immunoass...
1992-02-15 [Anal. Chem. 64(4) , 342-6, (1992)] |
|
Mono- and disalicylic acid derivatives: PTP1B inhibitors as ...
2007-10-15 [Bioorg. Med. Chem. 15 , 6535-48, (2007)] |
|
Effect of fluorine position on the coordinating ability of f...
2011-01-01 [J. Inorg. Biochem. 105(1) , 75-83, (2011)] |