| Structure | Name/CAS No. | Articles |
|---|---|---|
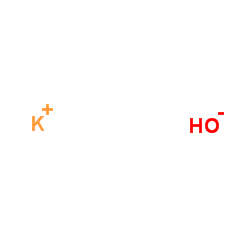 |
Potassium hydroxide
CAS:1310-58-3 |
|
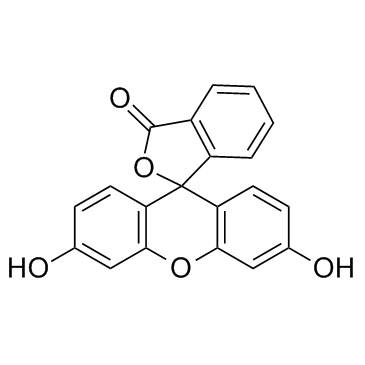 |
Fluorescein
CAS:2321-07-5 |
|
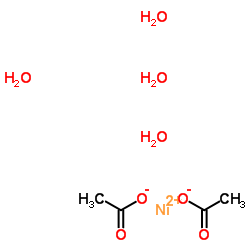 |
nickel acetate tetrahydrate
CAS:6018-89-9 |
|
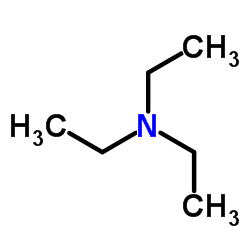 |
Triethylamine
CAS:121-44-8 |
|
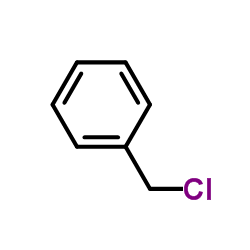 |
Benzyl chloride
CAS:100-44-7 |
|
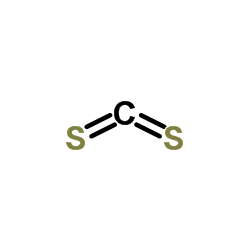 |
Carbon disulphide
CAS:75-15-0 |
|
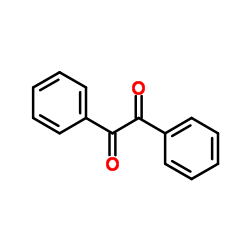 |
Benzil
CAS:134-81-6 |