| Structure | Name/CAS No. | Articles |
|---|---|---|
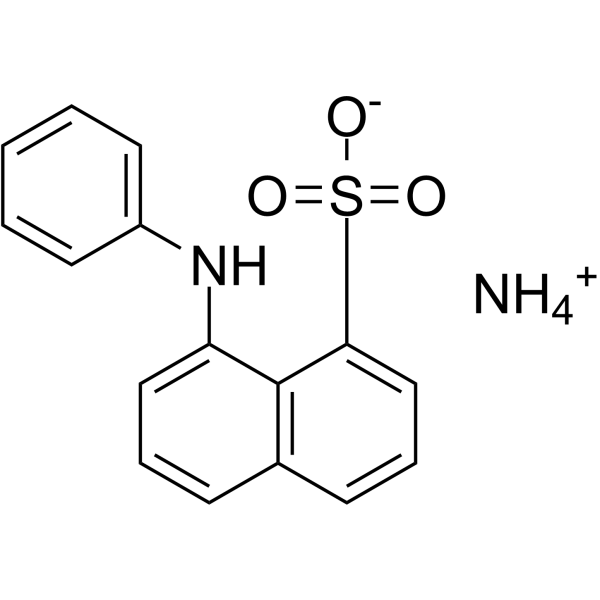 |
1-ANILINONAPHTHALENE-8-SULFONIC ACID AMMONIUM SALT
CAS:28836-03-5 |
|
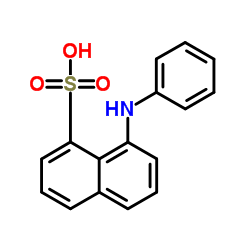 |
8-Anilino-1-naphthalenesulfonic acid
CAS:82-76-8 |
|
 |
Ananas comosus
CAS:37189-34-7 |
|
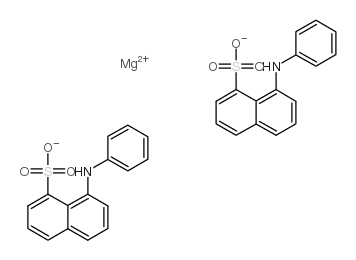 |
8-Anilino-1-naphthalenesulfonic acid magnesium salt
CAS:18108-68-4 |
|
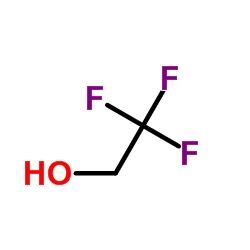 |
2,2,2-Trifluoroethanol
CAS:75-89-8 |