| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Methanol
CAS:67-56-1 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
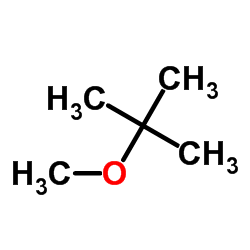 |
Methyl tert-butyl ether
CAS:1634-04-4 |
|
 |
trans-4-Hydroxycinnamic acid
CAS:501-98-4 |
|
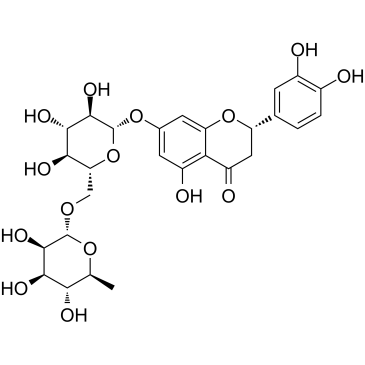 |
Eriocitrin
CAS:13463-28-0 |
|
 |
Phenol
CAS:108-95-2 |
|
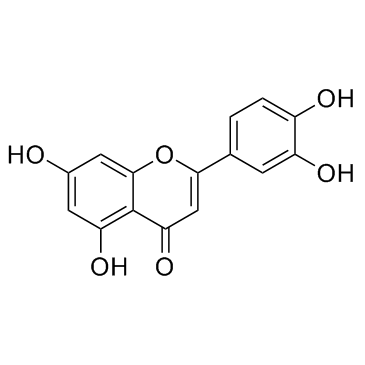 |
Luteolin
CAS:491-70-3 |
|
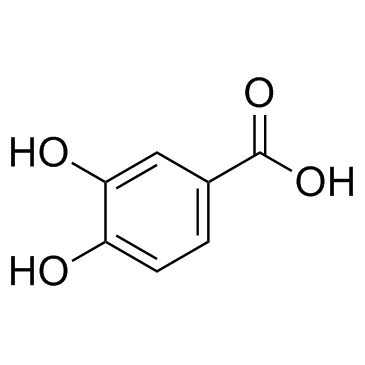 |
protocatechuic acid
CAS:99-50-3 |
|
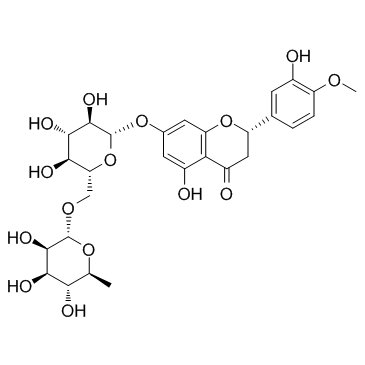 |
Hesperidin
CAS:520-26-3 |
|
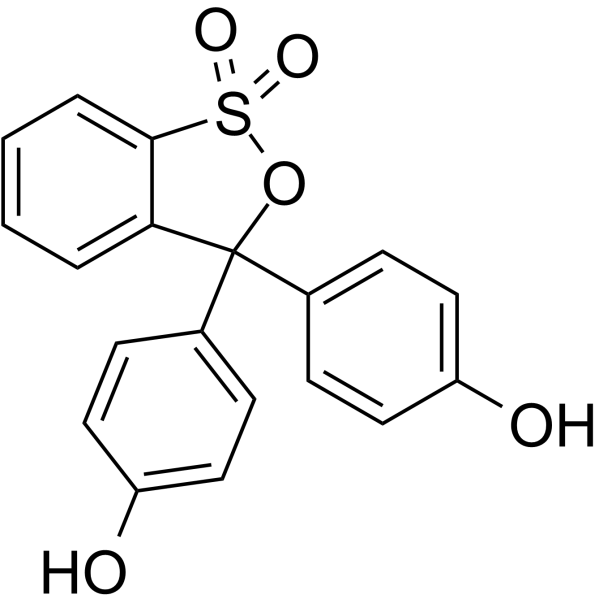 |
Phenol red
CAS:143-74-8 |