| Structure | Name/CAS No. | Articles |
|---|---|---|
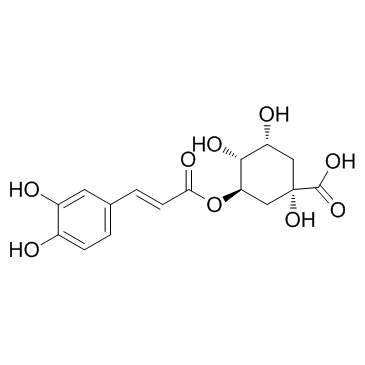 |
Chlorogenic acid
CAS:327-97-9 |
|
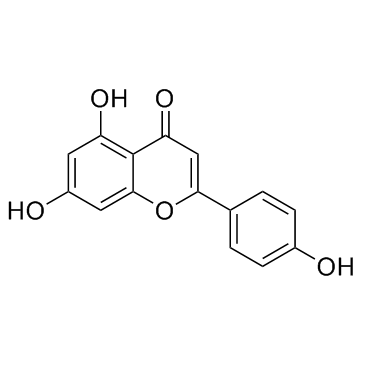 |
Apigenin
CAS:520-36-5 |
|
 |
trans-4-Hydroxycinnamic acid
CAS:501-98-4 |
|
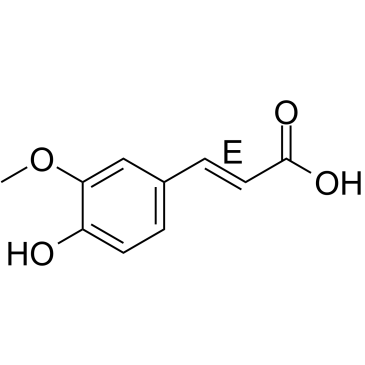 |
(E)-Ferulic acid
CAS:537-98-4 |
|
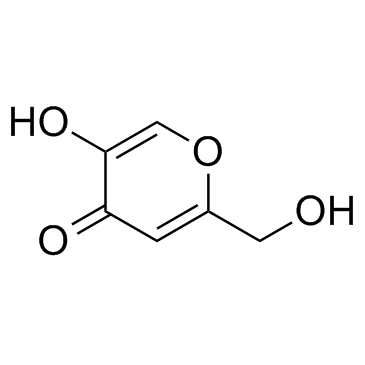 |
kojic acid
CAS:501-30-4 |
|
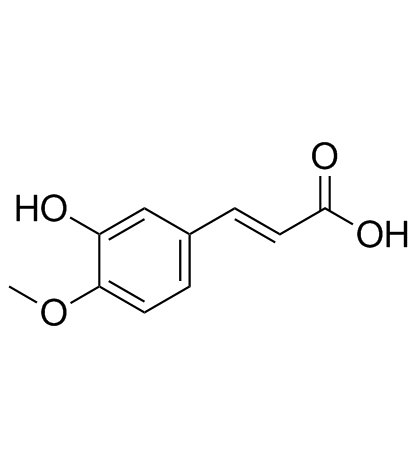 |
Isoferulic acid
CAS:537-73-5 |