| Structure | Name/CAS No. | Articles |
|---|---|---|
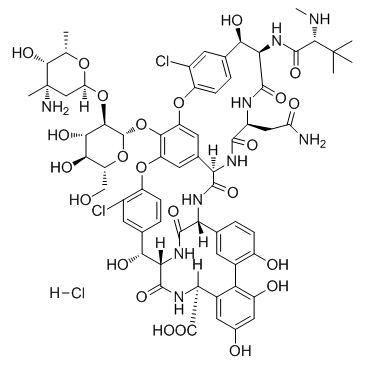 |
Vancomycin Hydrochloride
CAS:1404-93-9 |
|
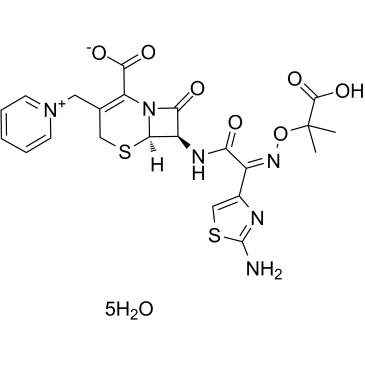 |
Ceftazidime Pentahydrate
CAS:78439-06-2 |
|
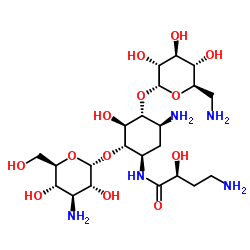 |
Amikacin
CAS:37517-28-5 |
|
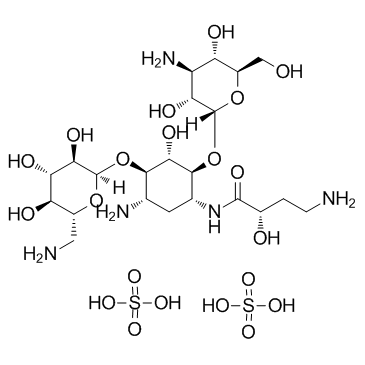 |
Amikacin sulfate
CAS:39831-55-5 |