| Structure | Name/CAS No. | Articles |
|---|---|---|
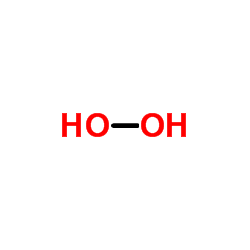 |
Hydrogen peroxide
CAS:7722-84-1 |
|
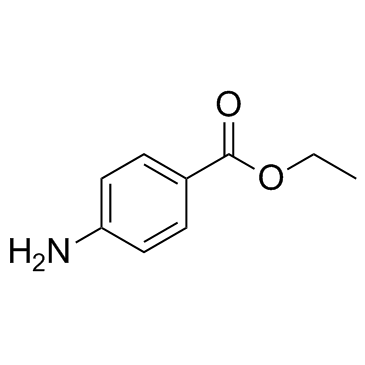 |
Benzocaine
CAS:94-09-7 |
|
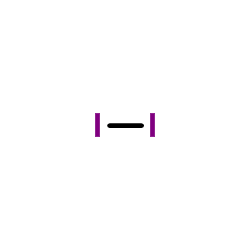 |
molecular iodine
CAS:7553-56-2 |
|
 |
Phenol
CAS:108-95-2 |
|
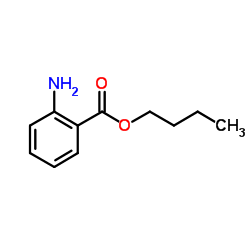 |
butyl anthranilate
CAS:7756-96-9 |
|
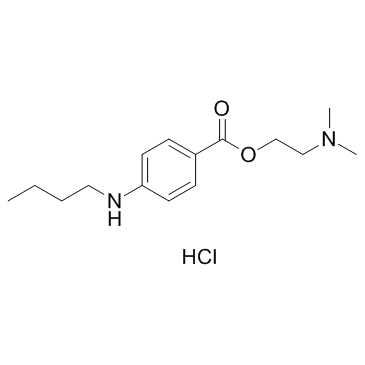 |
Tetracaine hydrochloride
CAS:136-47-0 |
|
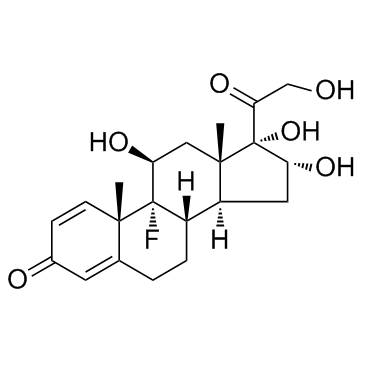 |
Triamcinolone
CAS:124-94-7 |
|
 |
Pyrrole
CAS:109-97-7 |