| Structure | Name/CAS No. | Articles |
|---|---|---|
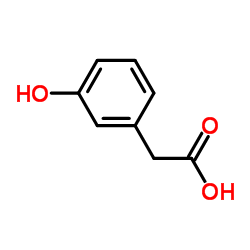 |
3-Hydroxyphenylacetic acid
CAS:621-37-4 |
|
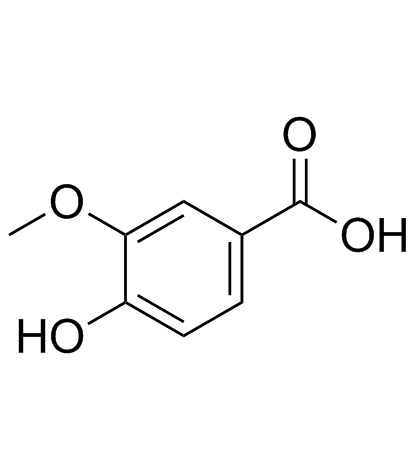 |
Vanillic acid
CAS:121-34-6 |
|
 |
Delphinidin chloride
CAS:528-53-0 |
|
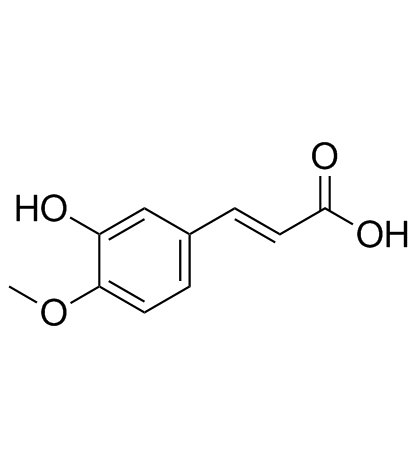 |
Isoferulic acid
CAS:537-73-5 |