| Structure | Name/CAS No. | Articles |
|---|---|---|
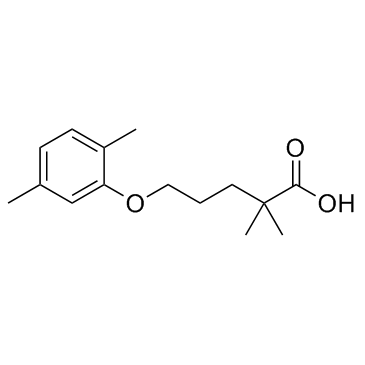 |
Gemfibrozil
CAS:25812-30-0 |
|
 |
Glimepiride
CAS:93479-97-1 |
|
 |
Repaglinide
CAS:135062-02-1 |