| Structure | Name/CAS No. | Articles |
|---|---|---|
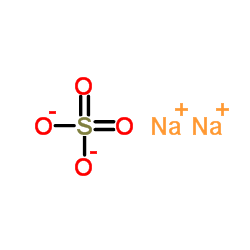 |
sodium sulfate
CAS:7757-82-6 |
|
 |
Sodium Oleate
CAS:143-19-1 |
|
 |
oleic acid
CAS:112-80-1 |
|
 |
Octadec-1-ene
CAS:112-88-9 |
|
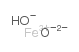 |
Iron hydroxide oxide
CAS:20344-49-4 |