| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
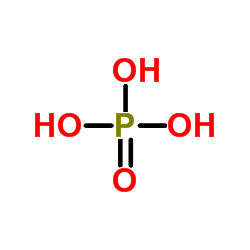 |
Phosphoric acid
CAS:7664-38-2 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Isopropanol
CAS:67-63-0 |
|
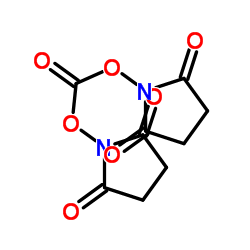 |
N,N'-Disuccinimidyl carbonate
CAS:74124-79-1 |
|
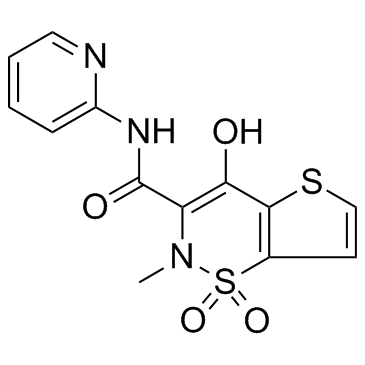 |
Tenoxicam
CAS:59804-37-4 |