| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
 |
Thiazole
CAS:288-47-1 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
 |
HEPES
CAS:7365-45-9 |
|
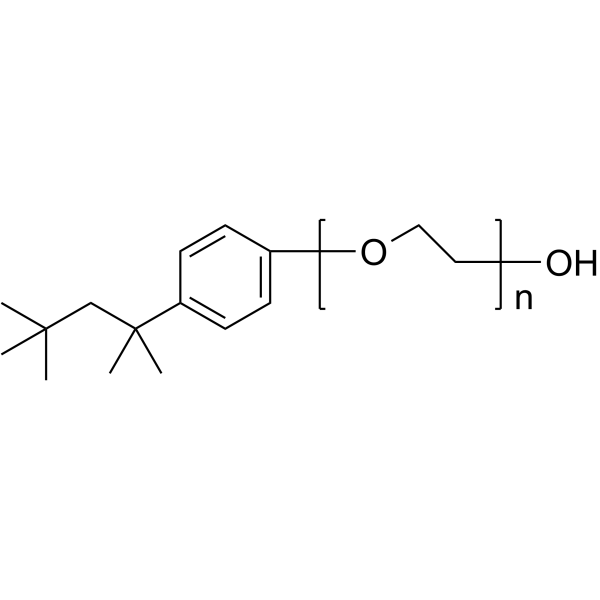 |
Triton X-100
CAS:9002-93-1 |
|
 |
Piperidine
CAS:110-89-4 |
|
 |
Diethyl ether
CAS:60-29-7 |
|
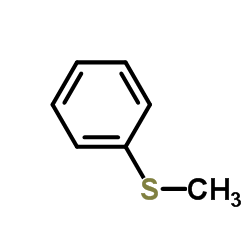 |
Thioanisole
CAS:100-68-5 |
|
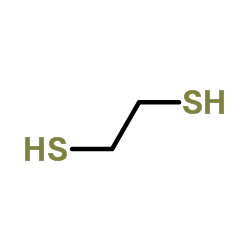 |
1,2-Ethanedithiol
CAS:540-63-6 |