| Structure | Name/CAS No. | Articles |
|---|---|---|
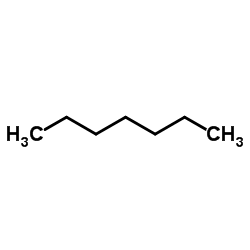 |
Heptane
CAS:142-82-5 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
 |
pentanol
CAS:71-41-0 |
|
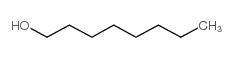 |
1-Octanol
CAS:111-87-5 |
|
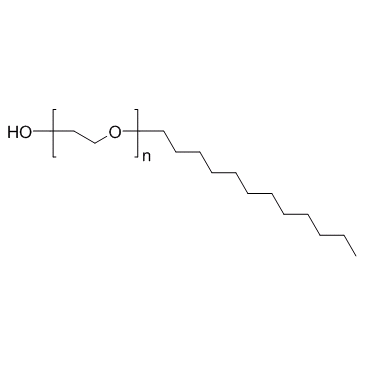 |
Polyoxyethylene lauryl ether
CAS:9002-92-0 |
|
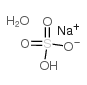 |
Sodium bisulfate monohydrate
CAS:10034-88-5 |