| Structure | Name/CAS No. | Articles |
|---|---|---|
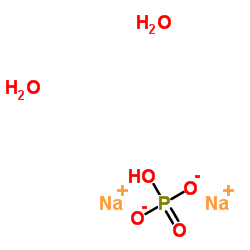 |
Sodium hydrogen phosphate hydrate (2:1:2)
CAS:10028-24-7 |
|
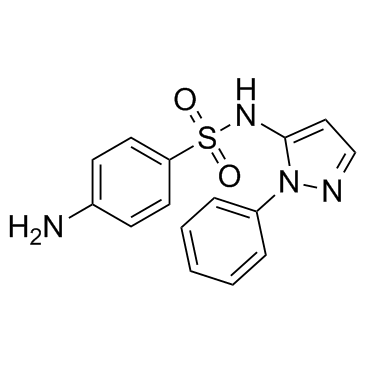 |
Sulfaphenazole
CAS:526-08-9 |
|
 |
Potassium
CAS:7440-09-7 |
|
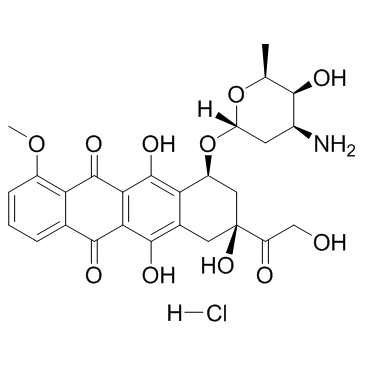 |
Doxorubicin Hydrochloride
CAS:25316-40-9 |
|
 |
Formic Acid
CAS:64-18-6 |
|
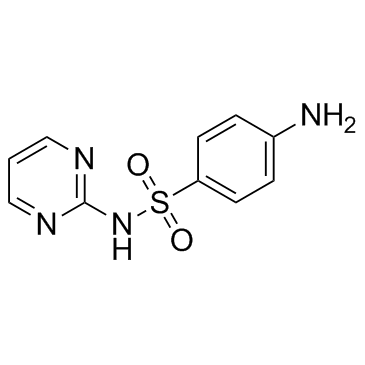 |
Sulfadiazine
CAS:68-35-9 |
|
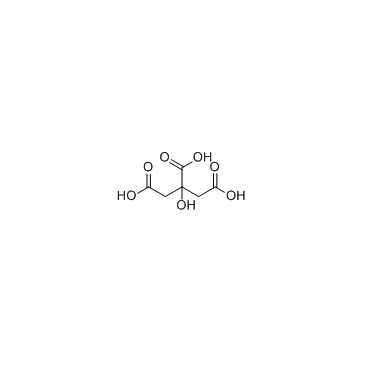 |
Citric Acid
CAS:77-92-9 |
|
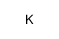 |
potassium hydride
CAS:7693-26-7 |
|
 |
Trimethoprim
CAS:738-70-5 |
|
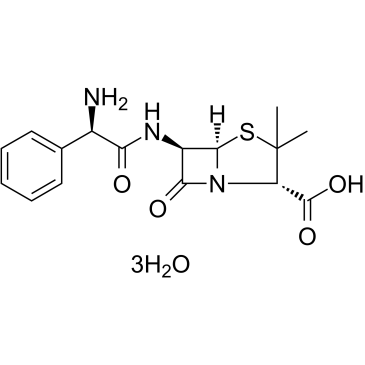 |
Ampicillin Trihydrate
CAS:7177-48-2 |