| Structure | Name/CAS No. | Articles |
|---|---|---|
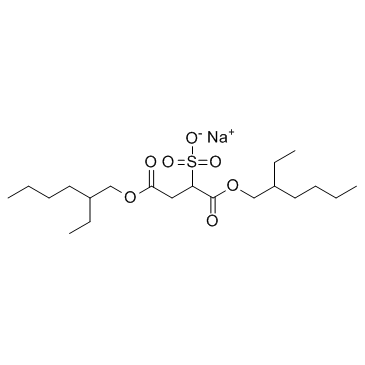 |
Docusate sodium
CAS:577-11-7 |
|
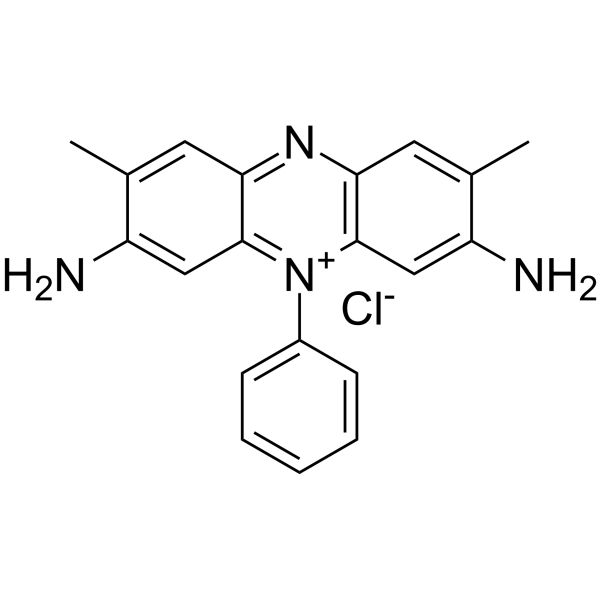 |
Safranin
CAS:477-73-6 |
|
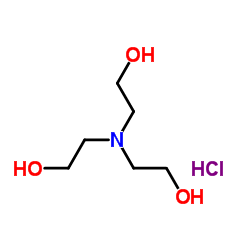 |
TRIETHANOLAMINE HYDROCHLORIDE
CAS:637-39-8 |
|
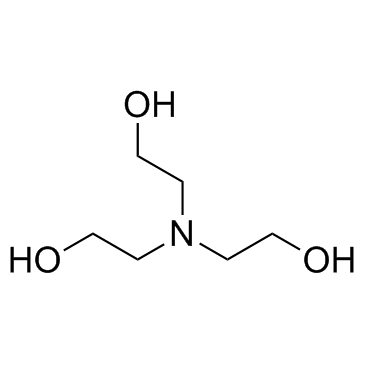 |
Triethanolamine
CAS:102-71-6 |