| Structure | Name/CAS No. | Articles |
|---|---|---|
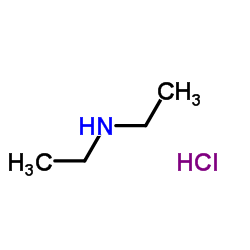 |
Diethylamine hydrochloride
CAS:660-68-4 |
|
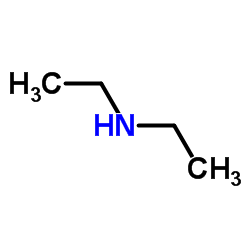 |
Diethylamine
CAS:109-89-7 |
|
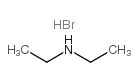 |
Diethylammonium bromide
CAS:6274-12-0 |