| Structure | Name/CAS No. | Articles |
|---|---|---|
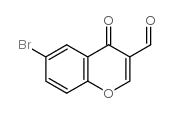 |
6-bromo-3-formylchromone
CAS:52817-12-6 |
|
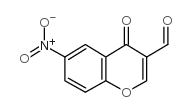 |
6-NITRO-4-OXO-4H-CHROMENE-3-CARBALDEHYDE
CAS:42059-80-3 |
|
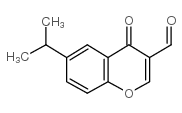 |
3-Formyl-6-isopropylchromone
CAS:49619-58-1 |
|
 |
6,8-Dibrom-4-oxo-4H-chromen-3-carbaldehyde
CAS:42059-76-7 |