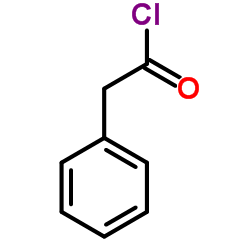
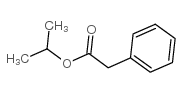
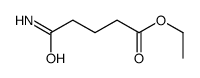
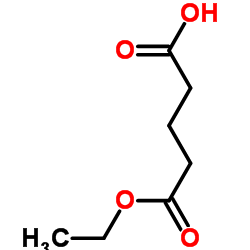
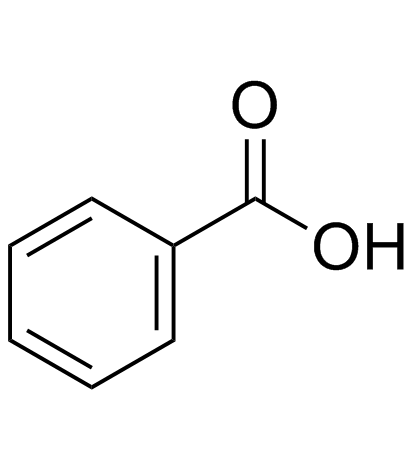
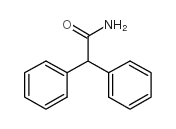
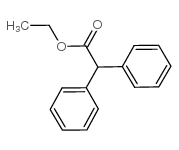
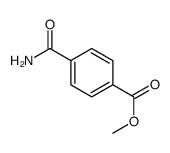
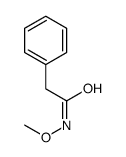
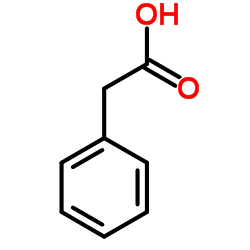
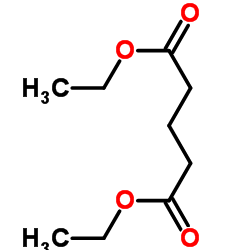
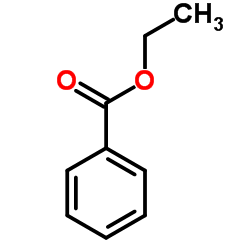
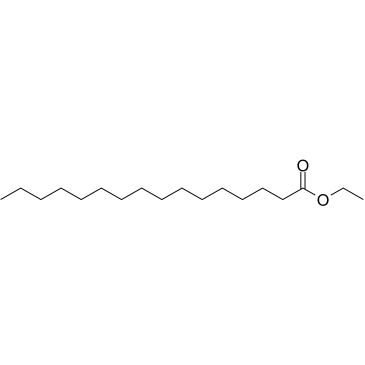
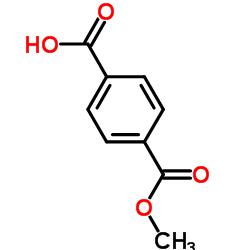
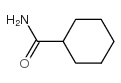
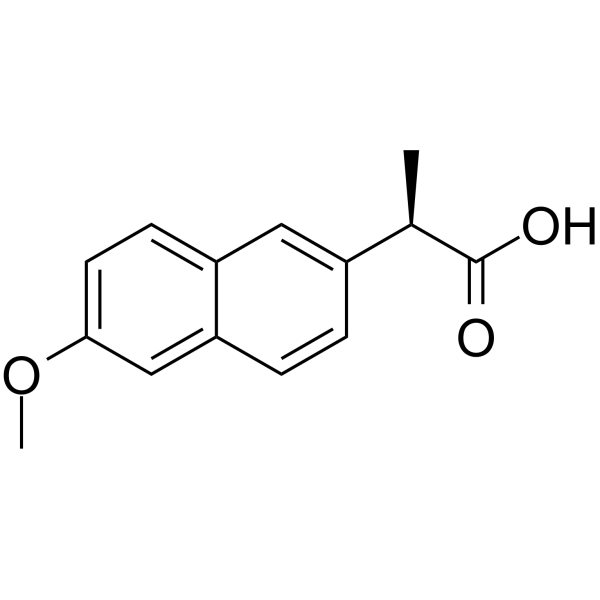
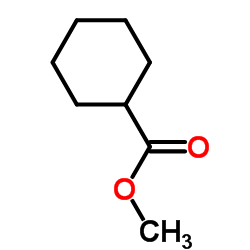
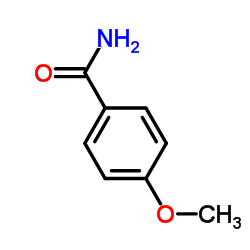
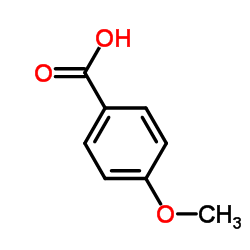
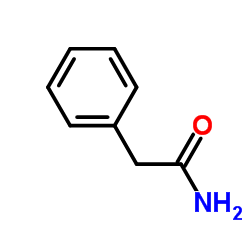
|
~% |
|
~% |
|
~% |
|
~90% |
|
~% |
|
~51% |
|
~80% |
|
~89% |
|
~90% |
|
~78% |
|
~% |
|
~75% |
|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~% |
|
~92% |
|
~93% |
|
~87% |
|
~86% |
|
~79% |
|
~87% |