Organic & Biomolecular Chemistry
2015-06-28
An iodine catalyzed metal free domino process for the stereoselective synthesis of oxygen bridged bicyclic ethers.
B V Subba Reddy, B Someswarao, N Prudhviraju, B Jagan Mohan Reddy, B Sridhar, S Kiran Kumar
Index: Org. Biomol. Chem. 13 , 6737-41, (2015)
Full Text: HTML
Abstract
A domino reaction has been developed for the synthesis of oxygen bridged bicyclic ethers through the coupling of 4-(2-hydroxyethyl)cyclohex-3-enols with aldehydes in the presence of 10 mol% of molecular iodine in dichloromethane at 25 °C. This method is highly diastereoselective affording the corresponding bicyclic ethers, i.e. octahydro-4a,7-epoxyisochromenes in good yields with high selectivity. It is the first report on the synthesis of oxygen bridged bicyclic ethers using a domino Prins strategy.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
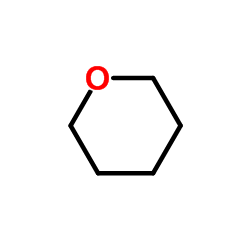 |
Tetrahydropyran
CAS:142-68-7 |
C5H10O |
Related Articles:
More...
|
Structurally and stereochemically diverse tetrahydropyran sy...
2010-04-12 [Angew. Chem. Int. Ed. Engl. 49(17) , 3069-72, (2010)] |
|
A SURGICAL APPROACH TO LARGE SUBRETINAL HEMORRHAGE USING PAR...
2015-08-01 [Retina (Philadelphia, Pa.) 35 , 1631-9, (2015)] |
|
Long-term heavy silicone oil intraocular tamponade.
2016-02-01 [Int. Ophthalmol. 36 , 07-Mar, (2016)] |
|
Is bond stretch isomerism in mononuclear transition metal co...
2015-07-28 [Dalton Trans. 44 , 12653-9, (2015)] |
|
Contribution of postsynaptic T-type calcium channels to para...
2016-02-15 [J. Physiol. 594 , 915-36, (2016)] |