Is bond stretch isomerism in mononuclear transition metal complexes a real issue? The misleading case of the MoCl5/tetrahydropyran reaction system.
Francesco Paolo Di Nicola, Massimiliano Lanzi, Fabio Marchetti, Guido Pampaloni, Stefano Zacchini
Index: Dalton Trans. 44 , 12653-9, (2015)
Full Text: HTML
Abstract
Distinct batches of orange (1a-e) and green crystals (2a-e) were isolated from the reactions of MoCl5 with tetrahydropyran (thp), respectively at room temperature (in CH2Cl2) and at ca. 80 °C (in ClCH2CH2Cl). Crystals 2a-e are isomorphous to 1a-e and the IR spectra are almost superimposable. 1a-e were identified by X-ray studies as cis-MoCl4(thp)2, in agreement with previous findings. Careful refinement of the X-ray data of 2a-e by modeling one position as disordered between chlorine (minor component) and oxygen (major component) led to the conclusion that 2a-e consisted of a mixture of cis-MoCl4(thp)2 and mer-MoOCl3(thp)2, the latter being largely prevalent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
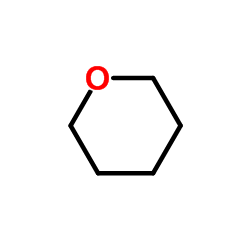 |
Tetrahydropyran
CAS:142-68-7 |
C5H10O |
|
Structurally and stereochemically diverse tetrahydropyran sy...
2010-04-12 [Angew. Chem. Int. Ed. Engl. 49(17) , 3069-72, (2010)] |
|
A SURGICAL APPROACH TO LARGE SUBRETINAL HEMORRHAGE USING PAR...
2015-08-01 [Retina (Philadelphia, Pa.) 35 , 1631-9, (2015)] |
|
Long-term heavy silicone oil intraocular tamponade.
2016-02-01 [Int. Ophthalmol. 36 , 07-Mar, (2016)] |
|
Contribution of postsynaptic T-type calcium channels to para...
2016-02-15 [J. Physiol. 594 , 915-36, (2016)] |
|
An iodine catalyzed metal free domino process for the stereo...
2015-06-28 [Org. Biomol. Chem. 13 , 6737-41, (2015)] |