Site-specific protonation microequilibria of penicillin and cephalosporin beta-lactam core molecules.
Kristóf Kóczián, Zoltán Szakács, József Kökösi, Béla Noszál
Index: Eur. J. Pharm. Sci. 32(1) , 1-7, (2007)
Full Text: HTML
Abstract
Acid-base chemistry of 6-aminopenicillanic acid (6-APA) and 7-aminocephalosporanic acid (7-ACA), the fundamental units of the two classical antibiotic families is characterised at the macroscopic and microscopic levels. (1)H NMR-pH and pH-potentiometric titrations were combined to monitor the previously unreported extent of the site-specific protonation of 6-APA and 7-ACA. Microscopic protonation constants were derived either from direct multiple fittings of NMR-pH titration curves of adjacent carbon-bound (1)H nuclei, using in situ pH-indicator molecules, or from newly synthesised methyl ester derivatives. The results indicate that even the amino protonation of both structures occurs well below pH 5, the major protonation route includes the zwitterionic forms, and protonation at the carboxylate site brings about a three-fold diminish in the amino basicity and vice versa.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
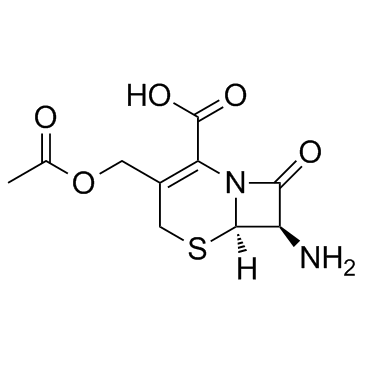 |
7-Aminocephalosporanic acid
CAS:957-68-6 |
C10H12N2O5S |
|
Construction of recombinant Escherichia coli D11/pMSTO and i...
2007-04-01 [J. Biotechnol. 129(3) , 400-5, (2007)] |
|
Batch production of deacetyl 7-aminocephalosporanic acid by ...
2004-08-01 [Appl. Microbiol. Biotechnol. 65(3) , 263-7, (2004)] |
|
A single Phe54Tyr substitution improves the catalytic activi...
2010-02-28 [New Biotechnology 27(1) , 78-84, (2010)] |
|
Double knockout of β-lactamase and cephalosporin acetyl este...
2012-06-01 [J. Biosci. Bioeng. 113(6) , 737-41, (2012)] |
|
Construction and application of fusion proteins of D-amino a...
2004-06-01 [Biotechnol. Lett. 26(11) , 939-45, (2004)] |