Batch production of deacetyl 7-aminocephalosporanic acid by immobilized cephalosporin-C deacetylase.
Akio Takimoto, Tomoaki Takakura, Hiroyoshi Tani, Shigeo Yagi, Kenji Mitsushima
Index: Appl. Microbiol. Biotechnol. 65(3) , 263-7, (2004)
Full Text: HTML
Abstract
Bacillus subtilis SHS0133 cephalosporin-C deacetylase (CAH) overexpressed in Escherichia coli was immobilized on an anion-exchange resin, KA-890, using glutaraldehyde. The activity yield of immobilized enzyme was approximately 55% of the free enzyme. The pH range for stability of the immobilized enzyme (pH 5-10) was broader than that for free enzyme. The K(m)(app) value of immobilized enzyme for 7-aminocephalosporanic acid (7-ACA) was similar to that of the free enzyme. This immobilized enzyme obeyed Michaelis-Menten kinetics similar to those of the free enzyme. A batch-type reactor with a water jacket was employed for deacetylation of 7-ACA using CAH immobilized on KA-890. Ten kilograms of 7-ACA were completely converted to deacetyl 7-ACA at pH 8.0 within 90 min. The reaction kinetics agreed well with a computer simulation model. Moreover, the immobilized enzyme exhibited only a slight loss of the initial activity even after repeated use (52 times ) over a period of 70 days. This reaction will thus be useful for the production of cephalosporin-type antibiotics.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
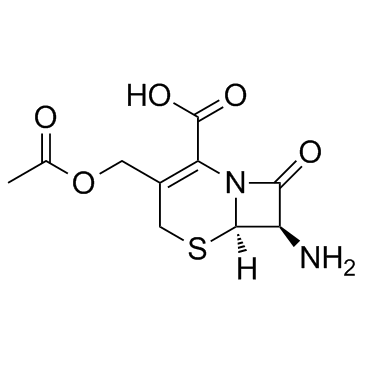 |
7-Aminocephalosporanic acid
CAS:957-68-6 |
C10H12N2O5S |
|
Construction of recombinant Escherichia coli D11/pMSTO and i...
2007-04-01 [J. Biotechnol. 129(3) , 400-5, (2007)] |
|
Site-specific protonation microequilibria of penicillin and ...
2007-09-01 [Eur. J. Pharm. Sci. 32(1) , 1-7, (2007)] |
|
A single Phe54Tyr substitution improves the catalytic activi...
2010-02-28 [New Biotechnology 27(1) , 78-84, (2010)] |
|
Double knockout of β-lactamase and cephalosporin acetyl este...
2012-06-01 [J. Biosci. Bioeng. 113(6) , 737-41, (2012)] |
|
Construction and application of fusion proteins of D-amino a...
2004-06-01 [Biotechnol. Lett. 26(11) , 939-45, (2004)] |