| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
N-hexane
CAS:110-54-3 |
|
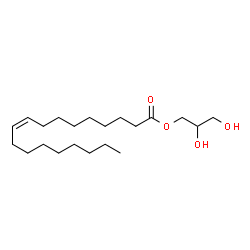 |
Monoolein
CAS:111-03-5 |
|
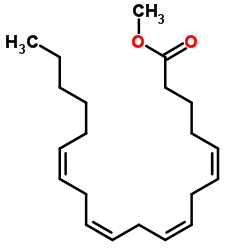 |
Methyl (5Z,8Z,11Z,14Z)-5,8,11,14-icosatetraenoate
CAS:2566-89-4 |
|
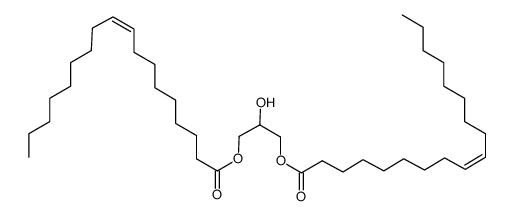 |
1,3-Dioleoyl Glycerol
CAS:2465-32-9 |
|
 |
2-Oleoylglycerol
CAS:3443-84-3 |