| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Diethyl ether
CAS:60-29-7 |
|
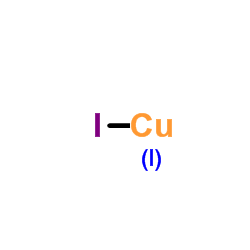 |
Copper(I) iodide
CAS:7681-65-4 |
|
![Dibenzo[b,d]furan Structure](https://image.chemsrc.com/caspic/103/132-64-9.png) |
Dibenzo[b,d]furan
CAS:132-64-9 |