| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Potassium bromide
CAS:7758-02-3 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
trifluoroacetic acid
CAS:76-05-1 |
|
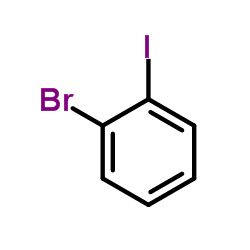 |
1-Bromo-2-iodobenzene
CAS:583-55-1 |
|
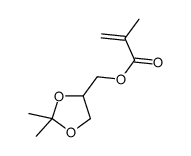 |
(2,2-dimethyl-1,3-dioxolan-4-yl)methyl methacrylate
CAS:7098-80-8 |
|
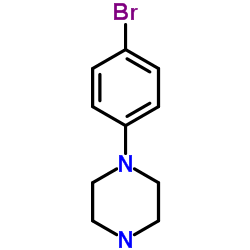 |
1-(4-Bromophenyl)piperazine
CAS:66698-28-0 |