| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
溴化钾
CAS:7758-02-3 |
|
 |
乙腈
CAS:75-05-8 |
|
 |
三氟乙酸(TFA)
CAS:76-05-1 |
|
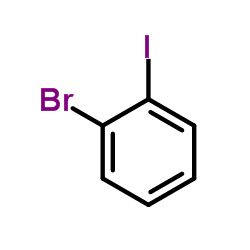 |
2-溴碘苯
CAS:583-55-1 |
|
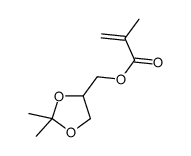 |
丙酮缩甘油异丁烯酸酯
CAS:7098-80-8 |
|
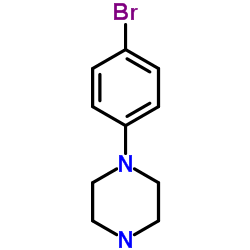 |
1-(4-溴苯基)哌嗪
CAS:66698-28-0 |