| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
stearic acid
CAS:57-11-4 |
|
 |
Fluorescein
CAS:2321-07-5 |
|
 |
Palmitic acid
CAS:57-10-3 |
|
 |
Arachidonic acid
CAS:506-32-1 |
|
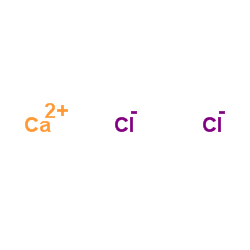 |
Calcium chloride
CAS:10043-52-4 |
|
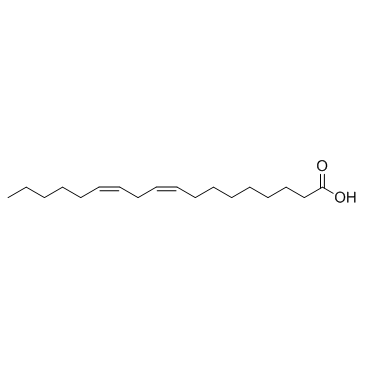 |
Linoleic acid
CAS:60-33-3 |
|
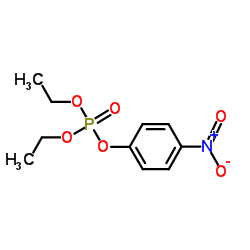 |
PARAOXON
CAS:311-45-5 |
|
 |
oleic acid
CAS:112-80-1 |
|
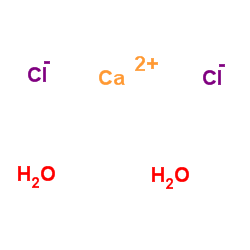 |
calcium chloride dihydrate
CAS:10035-04-8 |